ZINC PLATING
WHAT IS ZINC PLATING ?
Zinc and its compounds have been used for superfluous than 100 years as shielding and attractive layers
over a variety of metal substrates, mainly steel. Over the years, there has been a sum of processes
settled for applying zinc layers. The choice of which hangs on the substrate, coating requirements, and
cost. Of these, electroplating is the most dominant for handy and attractive applications.
When taking a zinc plating process, it is significant to know what processes are available and each of their certain advantages and disadvantages. Table 1 relates some of the more essential factors related to these methods.
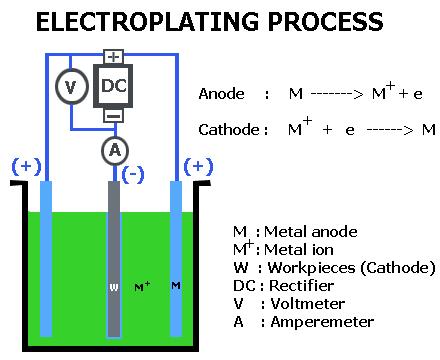
Why is zinc electroplating done?
The fundamental aim of atomic number 30 electroplating is to guard metals from rust or corrosion. atomic number 30 plated coatings are called putting to death coatings as a result of the atomic number 30 coating sacrifices itself to guard the underlying metal against corrosion. to guard metals like iron and steel against corroding, a method called atomic number 30 electroplating is employed. atomic number 30 electroplating needs to do with the electro-deposition of a skinny layer of atomic number 30 metal on the surface of another metal that is thought of as the substrate. The atomic number 30 coating is physical protection that prevents rust from moving the underlying metal surface. the explanation why atomic number 30 is chosen is its ability to fight corrosion.
In galvanization, atomic number 30 electroplating is the commonest and most widespread. For this reason, a third of all atomic number 30 metals is employed for electricity that is that the coating of a metal surface to guard such a metal against rust. atomic number 30 in most cases is exposed to the atmosphere, and as a result, atomic number 30 combines with the element within the wet atmosphere to supply oxide. Since the air found within the atmosphere is wet, atomic number 30 combines with the water within the air wet to supply atomic number 30 hydroxide. Likewise, the atomic number 30 hydroxide will mix with dioxide found within the atmosphere to supply a sealed and insoluble gray layer of atomic number 30 carbonate that sticks to the atomic number 30 at a lower place thereby protecting it from corroding. within the automobile industries, atomic number 30 plating is sometimes accustomed to preserve some essential elements of machines and vehicles like brake calipers, screws, pipes, and bolts. atomic number 30 plating is additionally employed in the electrical industries to guard electrical transmission instrumentality. atomic number 30 plating is additionally employed in the development of armories like armored tanks and guns.




Comments